There are millions of organic compounds known in this world. An organic compound is nothing but a class of chemical compound linked covalently with carbon atoms, generally hydrogen, nitrogen or oxygen. These compounds are associated with life processes also. The four major categories in living things are carbohydrates, proteins, lipids and nucleic acids. There are few carbons compounds like carbon dioxide, cyanite, cyanide which do not belong to organic compounds.
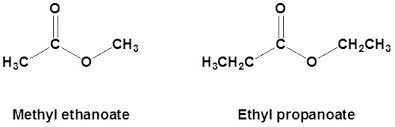
An organic compound has a formula RCOOR′, where R may be a hydrogen atom, an alkyl group, or an aryl group, and R′ may be an alkyl group or an aryl group but not a hydrogen atom is known as an ester. This compound was introduced in the 19th century by German chemist Leopold Gmelin. They are usually derived from carboxylic acids. Here is a detailed study of ester and its properties, uses etc.
Chemical and physical properties of ester
- Due to the weak intermolecular force, it smells partial.
- They are polar molecules like aldehydes and ketones. So they have dipole-dipole interaction and Van Der Waals dispersion forces.
- Lower esters are soluble relatively in water and increases as the chain length increases.
- Due to the absence of polar hydroxyl groups in esters than the parent acids, the boiling point will be more minor.
- They are comparatively less reactive than acid anhydride and acyl chlorides
- They react slowly to form amides and alcohols on reaction with ammonia
- Esters are reduced to primary alcohols in the presence of LiAlH4.
Preparation of esters
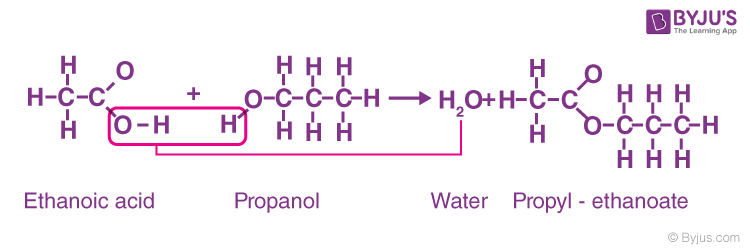
Esters are prepared by a reaction between alcohol and carboxylic acid in which the OH- group is released from the carboxylic acid molecule and H+ ion from the alcohol molecule. A water molecule and a molecule of an ester are formed as a byproduct.
CH3CH2COOH + CH3OH → CH3CH2COOCH3 + H2O
Esterification
During the formation of ester, a chemical reaction takes place, which is known as esterification. This can be defined as the process of combining an organic acid (RCOOH) with an alcohol (ROH) to form an ester (RCOOR) and water.
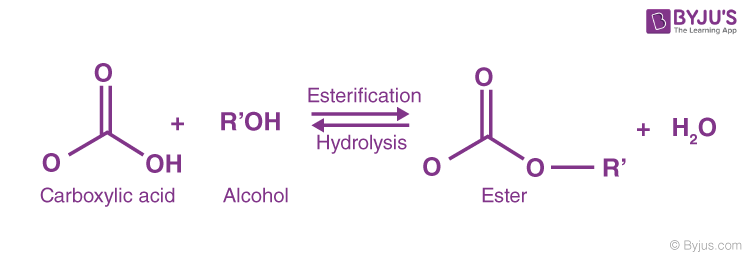
The three ways esterification can happen as,
- From acid anhydride and alcohol
- From acid chloride and alcohol
- From carboxylic acid and alcohol
Acid anhydride and alcohol
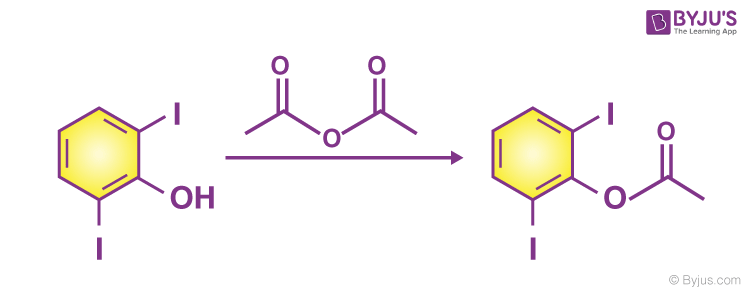
- Slow reaction take place in between acid anhydride and alcohol
- 2,6-diiodophenol reacts with an acid anhydride to form ester
Acid chloride and alcohol
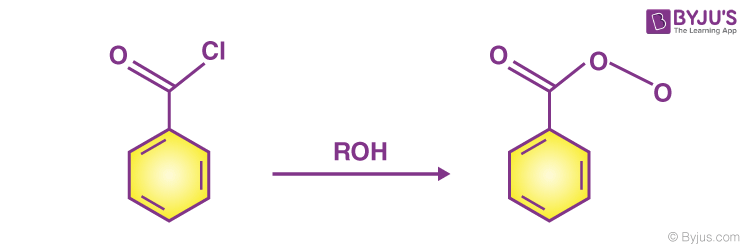
- At room temperature, acid chloride(benzoyl chloride) and alcohol react to produce an ester by liberating an acidic fume of hydrogen chloride.
Carboxylic acid and alcohol
Ethyl ethanoate or Ethyl acetate
Ethyl ethanoate is formed from the direct esterification of ethyl alcohol with acetic acid. The structure is given below.
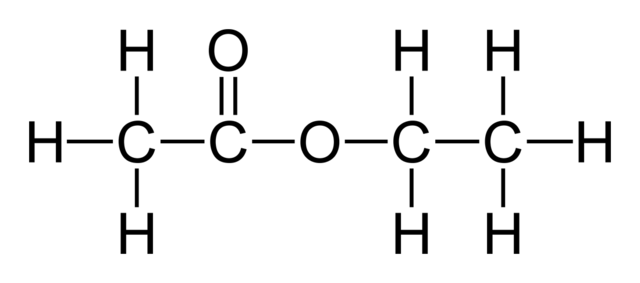
Ethyl acetate, also known as ethyl ethanoate, acts as a suitable solvent in many reactions. It is also used as an artificial flavour in ice creams and cakes. It is also found in alcoholic drinks like wines.
Uses of ester
- It is used as an organic solvent.
- They have fragrant odours and are used in certain perfumes.
- It is found in pheromones.
- Nitroglycerin is used as an explosive material.
- They are used as an artificial food flavouring substance.